Oxygen on Mars? A Chinese robot could search for the optimal production method on the red planet completely autonomously. Artificial intelligence should help with this.
Lots of carbon dioxide, a little water, solar energy and lots of rock – the conditions on Mars are not ideal. From a human perspective, oxygen is the main thing that is lacking. So how can oxygen be produced as efficiently as possible on the red planet ?
With artificial intelligence, says a Chinese research group. They have presented a robot in the journal “Nature Synthesis”. Thanks to artificial intelligence, the robot could work in a small laboratory on Mars in the future: It should find the perfect method to produce oxygen completely autonomously. Due to the great distance to Mars, the robot cannot be controlled in real time, but thanks to artificial intelligence, the robot could not only work completely independently, but also get better and better – that is the plan of the research team.
Robot searches for the perfect catalyst
To produce oxygen, the robot needs water above all. There is now increasing evidence that there are large amounts of water beneath the surface of Mars . Oxygen can be extracted from the water – using electricity from solar systems and with the perfect catalyst that makes the necessary chemical reaction possible .
This is where the robot with artificial intelligence comes into play. It is designed to produce the best catalyst from the Martian rock so that oxygen can be produced from the water. It is a so-called electro catalystthat is designed to use solar energy to initiate oxygen production.
In the search for the best catalyst, the robot mixes rock samples in different ways and uses them to develop new catalysts, which are then tested directly. How much oxygen is currently being produced? How can even more oxygen be produced? Using artificial intelligence, the robot nest the results and draws up new predictions and plans for new catalysts. Thanks to AI,it is constantly getting better.

First tests with Mars rock successful
The robot has now completed its first tests on Earth -including with real Martian rock that fell to Earth millions of years ago in the form of meteorites. The robot was given five different types of rock to test. Theoretically, this creates over 3.7 million possibilities for producing a catalyst.
A robot without artificial intelligence would need over 2,000 years to test all of them. But thanks to artificial intelligence, the robot does not have to go through all the possibilities and can find the perfect catalyst for oxygen production within weeks.
The Chinese research team has so far only experimented with robots in the laboratory. The robot and especially the small chemical laboratory still need to be developed for work on Mars. The experiments on Earth took place at minus 37 degrees to simulate the cold temperatures on Mars.In addition, even in the laboratory, the robot had to take into account that much less solar energy is available for the chemical reaction on Mars than on Earth.
NASA is already producing oxygen from carbon dioxide
The Chinese research team describes the experiment as a first proof of concept and wants to demonstrate new possibilities for producing oxygen. NASA currently has other plans. The US space agency is trying to produce oxygen from carbon dioxide using a pre-programmed robot.
95 percent of the carbon dioxide is in the atmosphere. The water, on the other hand, has to be extracted from the Martian soil at great expense. NASA already managed to produce oxygen with the Mars roverPerseverance in June 2023. The Moxie instrument produced 12 grams of oxygen within an hour during the test in June. NASA is now working on a larger instrument. artificial intelligence AI
The robot chemist spent six weeks working on Mars samples without any human intervention, creating 243 different molecules.
The robot chemist has produced compounds that have the potential to produce oxygen from water. Using artificial intelligence (AI), the robot analyzed Mars meteorites, as reported by space.com. Researchers believe this discovery will be beneficial for future human missions to Mars, where oxygen will be necessary for breathing and as rocket propellant, as further described in the report. Extracting oxygen from materials on Mars will eliminate the need to transport oxygen-producing materials from Earth.
The findings of the experiment have been detailed in the journal Nature Synthesis.
The scientists were inspired by the recent identification of substantial reserves of frozen water ice on the Martian surface.
The compounds generated by the robot chemist, known as catalysts, can initiate chemical reactions to separate water molecules and produce oxygen and hydrogen gas, according to space.com.
The meteorites from Mars on which the experiment was conducted were rocks that landed on Earth after being ejected from the Red Planet due to cosmic impacts.
After using a laser to scan the rocks, the AI-controlled robot identified over 3.7 million molecules that could be created from six different metallic elements in the rocks: iron, nickel, manganese, magnesium, aluminum, and calcium.
The robot chemist worked on the samples for six weeks without any human intervention and produced 243 different molecules. The most effective one it analyzed could separate water at -37 degrees Celsius, a temperature characteristic of Mars.
Jun Jiang, co-senior author of the study and a scientist at the University of Science and Technology of China in Hefei, told Space.com, “When I was young, I had dreams of interstellar exploration.”
A robot chemist has generated compounds that may be used to produce oxygen from water. The robot, powered by artificial intelligence (AI), examined meteorites from Mars, according to a report from space.com. Researchers believe this discovery will be beneficial for future human missions to Mars, where oxygen will be necessary for breathing and as rocket propellant, as described in the report. Extracting oxygen from materials on Mars will eliminate the need to transport oxygen-producing materials from Earth.
A study detailing the experiment has been published in the journal Nature Synthesis.
The scientists were inspired by the recent identification of substantial reserves of frozen water ice on the Martian surface.
The compounds generated by the robot chemist, known as catalysts, can initiate chemical reactions to separate water molecules and produce oxygen and hydrogen gas, according to space.com.
The meteorites from Mars on which the experiment was conducted were rocks that landed on Earth after being ejected from the Red Planet due to cosmic impacts.
After using a laser to scan the rocks, the AI-controlled robot identified over 3.7 million molecules that could be created from six different metallic elements in the rocks: iron, nickel, manganese, magnesium, aluminum, and calcium.
The robot chemist worked on the samples for six weeks without any human intervention and produced 243 different molecules. The most effective one it analyzed could separate water at -37 degrees Celsius, a temperature characteristic of Mars.
Jun Jiang, co-senior author of the study and a scientist at the University of Science and Technology of China in Hefei, told Space.com, “When I was young, I had dreams of interstellar exploration.”
“So when we finally saw that the catalysts made by the robot could actually produce oxygen by splitting water molecules, I felt like my dream was coming true. I even started to imagine that I, myself, will live on Mars in the future,” the scientists added.
According to scientists, identifying the best catalyst using conventional methods would have taken a human scientist 2,000 years.
One of the most significant hurdles to human interstellar travel is the inability to breathe in the depths of space. Oxygen is vital for life and is not as readily available as on Earth. With space agencies and researchers eyeing Mars exploration, the ability to generate oxygen for extended journeys is essential. Scientists have speculated about life on the red planet and also view it as a potential secondary planet for human habitation.
Researchers from the University of Science and Technology of China in Hefei have published a study about a robot chemist powered by artificial intelligence (AI). The robot’s objective is to extract water from Mars and convert it into oxygen.
According to one of the lead researchers, Jun Jiang, “We have developed a robotic AI system with a chemistry brain. We believe our machine can utilize compounds in Martian ores without human intervention.”
Creating oxygen on Mars is a significant challenge because it requires using only the resources available on the planet. A robot on Mars transforms meteorites into breathable air. Oxygen is a crucial starting point for this technology.
The research, published in Nature Synthesis, explains that a machine-learning model, utilizing both first-principles data and experimental measurements, can quickly and automatically identify the best catalyst formula from over three million possible compositions.
The study indicates that the robot chemist resolves two key challenges: the need for an unmanned synthesis system and the capability to identify the materials it is working with. AI robots are being explored as the preferred technology to address the Mars-oxygen problem.
Michael Hecht, from the Massachusetts Institute of Technology’s Haystack Observatory, was involved in the Mars Oxygen In-Situ Resource Utilization Experiment (MOXIE). He notes that the robot was able to produce small amounts of oxygen in the predominantly carbon dioxide Martian atmosphere during a 2021 mission. Although the current output is minimal, there is potential for augmentation.
An autonomous robotic chemist in a lab has developed an oxygen-producing catalyst from minerals found in Martian meteorites. This process could potentially provide oxygen for astronauts on Mars in the future.
Transporting supplies to a future Martian colony via spacecraft would be highly costly, making the utilization of Mars’s natural resources an attractive option. However, this can be challenging due to the limited elements available on Mars compared to Earth.
Yi Luo and colleagues at the University of Science and Technology of China in Hefei have created a fully automated robot chemist. The machine used a high-powered laser to analyze the chemical composition of five Martian meteorites and identified six elements in notable quantities: iron, nickel, calcium, magnesium, aluminum, and manganese.
“On Earth, we don’t use these six elements because we have more choice,” says Luo. “These six elements are not the best for this kind of catalyst and it limits its performance, but it’s what you’ve got on Mars.”
There are over 3.7 million different combinations of Martian elements, which would take over 2000 years to test manually if each round of testing took around 5 hours, according to Luo.
Instead of testing every combination, the robot utilizes artificial intelligence to predict the best catalyst combination for oxygen production. It then tested over 200 catalysts, utilizing a briny solution and carbon dioxide as the raw materials.
The robot ultimately identified a catalyst comparable to the best available catalysts on Earth from a decade ago, according to Luo. This catalyst can function at −37°C (−35°F), similar to temperatures on Mars, for over six days continuously. Luo and the team calculated that a 3-metre high, 100-square-metre room on Mars equipped with this catalyst on its ceiling could produce oxygen levels comparable to those on Earth in about 15 hours.
“Getting [the robot] to work is a significant achievement, as it requires getting numerous components to function together,” states Ross King from the University of Cambridge. While it might be easier to design materials on Earth and transport them to Mars in certain cases, autonomous robot chemists could be crucial for exploring farther into the solar system, where communication is more challenging.
Researchers hope that a scaled-up version could one day produce enough oxygen to sustain humans on Mars.
A lunchbox-sized instrument succeeded in producing breathable oxygen on Mars, performing the function of a small tree.
Since last February, the Mars Oxygen In-Situ Resource Utilization Experiment (MOXIE) has been effectively creating oxygen from the carbon dioxide-rich atmosphere of the red planet.
Research suggests that an expanded version of MOXIE could be dispatched to Mars to continuously generate oxygen at a rate equivalent to several hundred trees, ahead of human visits to the planet.

MOXIE was part of Nasa’s Perseverance rover mission, landing on the Martian surface.
According to a study, by the end of 2021, MOXIE was able to produce oxygen in seven experimental runs, under different atmospheric conditions, including day and night, and across various Martian seasons.
In each run, it achieved the goal of producing 6g of oxygen per hour – a rate similar to a modest tree on Earth.
The system is envisioned to have the capacity to generate enough oxygen to sustain humans once they reach Mars and to fuel a rocket for the return journey to Earth.
Moxie’s deputy principal investigator, Jeffrey Hoffman, a professor at the Massachusetts Institute of Technology’s Department of Aeronautics and Astronautics, stated: “This is the initial demonstration of utilizing resources on the surface of another planetary body and altering them chemically to produce something useful for a human mission.”
The current model of the device is intentionally small to fit on the Perseverance rover and operates for brief periods. A full-scale oxygen production facility would feature larger units designed to operate continuously.
Moxie has proven its ability to produce oxygen at various times during the Martian day and year. Michael Hecht, the principal investigator of the Moxie mission at MIT’s Haystack Observatory, commented: “The only remaining step is to demonstrate its operation at dawn or dusk, when temperatures change significantly. We have a solution that will enable us to achieve this, and once tested in the lab, we can reach that final milestone.”
If the system can function effectively despite frequent on and off cycles, it suggests that a full-scale system designed for continuous operation could function for thousands of hours.
Hoffman noted: “To support a human mission to Mars, we have to bring a lot of stuff from Earth, such as computers, spacesuits, and habitats. But producing oxygen on-site? If it’s feasible, then go for it – you’re way ahead of the game.”
The initial experiment to produce oxygen on another planet has concluded on Mars, surpassing NASA’s original objectives and demonstrating capabilities that could benefit future astronaut missions.
The Mars Oxygen In-Situ Resource Utilization Experiment (MOXIE), a microwave-sized device, is located on the Perseverance rover. The experiment began over two years ago, a few months after the rover landed on Mars. Since then, MOXIE has generated 122 grams of oxygen, equivalent to the amount a small dog breathes in 10 hours, according to NASA. The instrument converts some of Mars’ abundant carbon dioxide into oxygen.
During its peak efficiency, MOXIE produced 12 grams of oxygen per hour at 98% purity or higher, doubling NASA’s goals for the instrument. On August 7, MOXIE completed its 16th and final run, fulfilling all its requirements.
“We are delighted to have supported a breakthrough technology like MOXIE that could convert local resources into useful products for future exploration missions,” said Trudy Kortes, director of technology demonstrations at NASA’s Space Technology Mission Directorate. “By validating this technology in real-world conditions, we have moved one step closer to a future where astronauts can ‘live off the land’ on the Red Planet.”
Implications of MOXIE
The Martian atmosphere is 96% carbon dioxide, which is not suitable for oxygen-breathing humans. MOXIE functions by splitting up carbon dioxide molecules, containing one carbon atom and two oxygen atoms, separating the oxygen molecules and emitting carbon monoxide as a byproduct. The instrument’s system analyzes the purity and quantity of the oxygen as the gases pass through it.
The device was constructed using heat-tolerant materials, such as a coat of gold and aerogel, as the conversion process necessitates temperatures of up to 1,470 degrees Fahrenheit (798 degrees Celsius). These materials prevented heat from dissipating and damaging any part of the rover.
An efficient carbon dioxide to oxygen conversion system could have various benefits. Enhanced versions of devices like MOXIE in the future could supply breathable air for life support systems and convert and store oxygen required for rocket fuel for a return trip to Earth.
“MOXIE’s impressive performance proves that extracting oxygen from Mars’ atmosphere is feasible, oxygen that could help provide breathable air or rocket propellant for future astronauts,” said NASA Deputy Administrator Pam Melroy. “Developing technologies to utilize resources on the Moon and Mars is crucial for establishing a long-term lunar presence, creating a robust lunar economy, and enabling the initial human exploration campaign to Mars.”
Transporting thousands of pounds of rocket propellant and oxygen from Earth to Mars on the initial trip would be immensely challenging and expensive, leaving less room for other necessities on the spacecraft. Technologies like MOXIE could enable astronauts to live off the land and harness local resources.
Lessons from the small MOXIE experiment can now be applied to develop a full-scale system that incorporates an oxygen generator capable of liquefying and storing the oxygen. The next major step is to test other technologies on Mars that could further exploration, such as tools and habitat materials.
“We need to prioritize which technologies to validate on Mars,” stated Michael Hecht, principal investigator of MOXIE at the Massachusetts Institute of Technology. “Many technologies are on the validation list, and I’m glad that MOXIE was the first.”
Despite the seeming distance, efforts have recently intensified to prepare for human habitation on Mars, including training for astronauts and settlers, as well as the development of new technologies to support them during their mission. The unveiling of an AI-powered “robot chemist” by a group of researchers in China this week brings us closer to establishing this support system.
To provide some context about Mars, NASA’s Curiosity rover discovered evidence in October suggesting that Mars was once a “planet of rivers” with flowing water that might have supported life. Furthermore, the presence of solid water, or ice, on the planet’s surface has been known for some time, particularly in polar ice caps and Martian soil. In 2022, Cambridge University presented evidence suggesting the existence of liquid water beneath the ice caps.
The significance of water on Mars is due in part to its oxygen content, which is scarce in the Martian atmosphere, posing a challenge for future habitation. As a result, extracting oxygen is likely necessary for the survival of astronauts and space settlers on the planet. This is where a team of scientists, led by Jun Jiang at Hefei’s University of Science and Technology of China, comes into play.
The team emphasizes in their recent study, published in Nature Synthesis, that “Oxygen supply must be the top priority for any human activity on Mars, because rocket propellants and life support systems consume substantial amounts of oxygen.” However, continuously ferrying oxygen tanks or extraction tools to and from Mars is impractical and expensive, necessitating in-situ oxygen extraction. The team claims to have found a solution involving Martian meteorites, an innovative robot, and AI.
According to the study, the team developed a robot capable of using materials found on Mars to create catalysts that facilitate the breakdown of water, releasing oxygen in the process, and capturing it for various uses. The system is designed to operate autonomously, without human intervention.
“We have created a robotic AI system with a chemistry brain,” comments Jiang to Nature. “We believe that our machine can utilize compounds in Martian ores without human guidance.” With its machine-learning model “brain” and robotic arm, the system is purportedly able to produce nearly 60 grams of oxygen per hour for every square meter of Martian material. Although this may seem modest, Jiang emphasizes that “The robot can work continuously for years.”
The researchers substantiated their claims by using the robot to process meteorites originating from Mars, or that simulated the planet’s surface, demonstrating its ability to independently carry out several steps, such as dissolving, separating, and analyzing the material. Additionally, the robot searched more than 3.7 million formulae to identify a chemical that could break down water, a task estimated to take a human researcher around 2,000 years.
This does not necessarily imply that simpler methods of synthesizing oxygen on Mars will not be developed before human habitation. NASA’s MOXIE, for example, demonstrated a method of extracting oxygen from the Martian atmosphere, which is primarily carbon dioxide. Although MOXIE’s oxygen production has been limited so far, it is believed that with a more powerful power source, it could efficiently produce enough oxygen to support a human settlement.
Regardless of future developments, Jiang’s robot chemist has broader applications than just oxygen production. The AI has the potential to learn and produce various useful catalysts, creating a range of beneficial chemicals from Martian materials, such as fertilizers. Moreover, it could transfer its knowledge and applications to other celestial bodies, including the moon and beyond.
NASA has achieved another milestone in its latest Mars mission by successfully converting carbon dioxide from the Martian atmosphere into pure, breathable oxygen, as announced by the US space agency on Wednesday.
This remarkable feat, conducted by an experimental device named MOXIE (Mars Oxygen In-Situ Resource Utilization Experiment) aboard the Perseverance rover, took place on Tuesday. This toaster-sized instrument produced approximately 5 grams of oxygen in its initial activation, equivalent to roughly 10 minutes’ worth of breathing for an astronaut, according to NASA.
Though the initial outcome was unimpressive, the accomplishment signified the first experimental extraction of a natural resource from another planet’s environment for direct human use.
“MOXIE isn’t simply the first tool to create oxygen on a different world,” remarked Trudy Kortes, head of technology demonstrations at NASA’s Space Technology Mission Directorate. She characterized it as the first technology of its kind to support future missions in “living off the land” of another planet.
The device operates using electrolysis, a process that utilizes high temperatures to separate oxygen atoms from carbon dioxide molecules, which make up about 95% of Mars’ atmosphere.
The remaining 5% of Mars’ atmosphere, which is only about 1% as dense as Earth’s, consists mainly of molecular nitrogen and argon. Oxygen is present in negligible trace amounts on Mars.
However, an ample supply is considered crucial for eventual human exploration of the Red Planet, serving as a sustainable source of breathable air for astronauts and as a necessary component for rocket fuel to transport them back home.
The quantities needed for launching rockets from Mars are especially challenging.
According to NASA, launching four astronauts from the Martian surface would require around 15,000 pounds (7 metric tons) of rocket fuel, combined with 55,000 pounds (25 metric tons) of oxygen.
Bringing a one-ton oxygen-conversion device to Mars is more feasible than attempting to transport 25 tons of oxygen in tanks from Earth, as mentioned by MOXIE principal investigator Michael Hecht of the Massachusetts Institute of Technology in NASA’s press release.
Astronauts living and working on Mars might collectively require approximately one metric ton of oxygen to last an entire year, remarked Hecht.
MOXIE is designed to produce up to 10 grams per hour as a proof of concept, and scientists plan to operate the machine at least nine more times over the next two years under varying conditions and speeds, as stated by NASA.
The first oxygen conversion run occurred a day after NASA accomplished the historic first controlled powered flight of an aircraft on another planet with the successful takeoff and landing of a small robotic helicopter on Mars.
Similar to MOXIE, the twin-rotor helicopter named Ingenuity hitched a ride to Mars with Perseverance, whose primary mission is to search for evidence of ancient microbial life on Mars.
On Mars’ red and dusty surface, an instrument the size of a lunchbox is demonstrating its ability to reliably replicate the functions of a small tree.
The MIT-led Mars Oxygen In-Situ Resource Utilization Experiment, or MOXIE, has been effectively generating oxygen from the carbon dioxide-rich atmosphere of the Red Planet since April 2021, approximately two months after its arrival on the Martian surface as part of NASA’s Perseverance rover and Mars 2020 mission.
In a study released today in the journal Science Advances, researchers disclose that, by the end of 2021, MOXIE managed to produce oxygen in seven experimental runs, in various atmospheric conditions, including during the day and night, and across different Martian seasons. During each run, the instrument achieved its target of generating six grams of oxygen per hour—a rate similar to that of a modest tree on Earth.
Researchers envision that an enlarged version of MOXIE could be dispatched to Mars before a human mission to continuously generate oxygen at a rate equivalent to several hundred trees. At this capacity, the system should produce enough oxygen to sustain humans upon their arrival and fuel a rocket for returning astronauts to Earth.
Thus far, MOXIE’s consistent output is a promising initial step toward that objective.
“We have gained a wealth of knowledge that will guide future systems on a larger scale,” remarked Michael Hecht, principal investigator of the MOXIE mission at MIT’s Haystack Observatory.
MOXIE’s oxygen production on Mars also signifies the first demonstration of “in-situ resource utilization,” the concept of harvesting and using the materials of a planet (in this case, carbon dioxide on Mars) to generate resources (such as oxygen) that would otherwise need to be transported from Earth.
“This is the initial demonstration of actually utilizing resources on the surface of another planetary body and chemically transforming them into something beneficial for a human mission,” noted MOXIE deputy principal investigator Jeffrey Hoffman, a professor in MIT’s Department of Aeronautics and Astronautics. “In that sense, it’s a historic achievement.”
MIT co-authors of Hoffman and Hecht’s, including MOXIE team members Jason SooHoo, Andrew Liu, Eric Hinterman, Maya Nasr, Shravan Hariharan, Kyle Horn, and Parker Steen, as well as collaborators from various institutions, including NASA’s Jet Propulsion Laboratory, which oversaw MOXIE’s development, flight software, packaging, and pre-launch testing, also contributed to the study.
The current MOXIE version is intentionally small to fit on the Perseverance rover and is designed to operate for short periods based on the rover’s exploration schedule and mission responsibilities. In contrast, a full-scale oxygen factory would consist of larger units running continuously.
Despite the necessary design compromises, MOXIE has demonstrated its ability to efficiently convert Mars’ atmosphere into pure oxygen reliably. It begins by filtering Martian air to remove contaminants, pressurizing the air, and then passing it through the Solid Oxide Electrolyzer (SOXE), an instrument developed and built by OxEon Energy. The SOXE electrochemically splits the carbon dioxide-rich air into oxygen ions and carbon monoxide.
The oxygen ions are isolated and recombined to form breathable molecular oxygen (O2), which MOXIE measures for quantity and purity before releasing it back into the air along with carbon monoxide and other atmospheric gases.
Since its landing in February 2021, the MOXIE engineers have activated the instrument seven times throughout the Martian year. Each activation took a few hours to warm up, followed by an hour to produce oxygen before being powered down. The activations were scheduled for different times of the day or night and in different seasons to test MOXIE’s adaptability to the planet’s atmospheric conditions.
Mars’ atmosphere is more variable than Earth’s, with air density varying by a factor of two and temperatures fluctuating by 100 degrees throughout the year. The objective is to demonstrate that MOXIE can operate in all seasons.
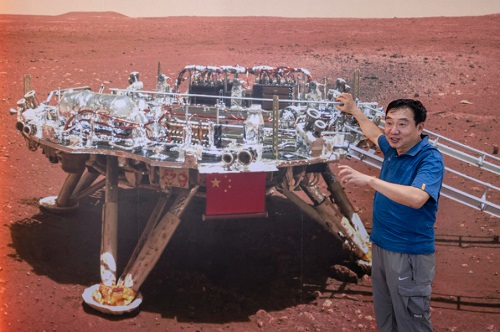
So far, MOXIE has proven its ability to produce oxygen at almost any time of the Martian day and year.
The only untested scenario is running at dawn or dusk when the temperature changes significantly. The team is confident that they have a solution and once tested in the lab, they can demonstrate the ability to run MOXIE at any time.
Looking ahead, as MOXIE continues to produce oxygen on Mars, the engineers plan to increase its production capacity, especially in the Martian spring when atmospheric density and carbon dioxide levels are high.
The upcoming run will take place during the highest atmospheric density of the year, aiming to produce as much oxygen as possible. The system will be set to run at maximum levels, pushing its limits while monitoring for signs of wear and tear. As MOXIE is only one of several experiments on the Perseverance rover and cannot run continuously, successful intermittent operation could indicate its potential for continuous operation in a full-scale system.
To support a human mission to Mars, it is crucial to produce oxygen on-site, as the transportation of oxygen from Earth is not practical, unlike other essentials such as computers, spacesuits, and habitats. Therefore, the successful operation of MOXIE is a significant step forward in this endeavor.
NASA designed a device called MOXIE to produce oxygen from the carbon dioxide found in the Martian atmosphere. This instrument works using a process known as electrolysis, which uses high heat to separate oxygen atoms from carbon dioxide molecules.
Carbon dioxide makes up about 95 percent of the Martian atmosphere, with the remaining portion mainly composed of molecular nitrogen and argon. Only 0.16 percent of the Martian atmosphere consists of molecular oxygen.
For future exploration and potential human habitation of Mars, a substantial oxygen supply is necessary for breathing and producing rocket fuel for launches from the Martian surface. NASA funded the MOXIE experiment, developed by a team from the Massachusetts Institute of Technology (MIT) and carried to Mars onboard the Perseverance rover.
MOXIE successfully converted carbon dioxide from the Martian atmosphere into oxygen during its first test in April 2021, producing 5.4 grams of oxygen in one hour. Subsequent experiments were conducted to assess the system’s effectiveness.
Earlier this month, organizers of the test project announced that MOXIE had finished its 16th and final experiment. They highlighted the device’s “impressive performance” as proof that extracting oxygen from the Martian atmosphere is feasible. This oxygen could potentially be used to provide breathable air or rocket propellant for future astronauts, the statement explained.
According to NASA, MOXIE has generated a total of 122 grams of oxygen since Perseverance landed on Mars, equivalent to what a small dog would breathe in 10 hours. Although the oxygen amount is small, it signifies the first experimental extraction of a natural resource from another planet’s environment.
When operating at peak efficiency, the instrument was capable of producing 12 grams of oxygen per hour, twice the initial estimate by NASA engineers.
The MOXIE team has also been evaluating the oxygen purity produced by the device, reporting that it was consistently over 98% pure.
The latest Mars experiments with MOXIE are aiming at helping NASA develop a significantly larger version of the system, which could potentially be deployed on Mars in the future.
According to NASA’s description of the instrument, the objective of a larger MOXIE would be to generate and store all the oxygen needed for astronauts and their rocket before they embark on their mission. The space agency noted that such a system would need to produce between 2,000 to 3,000 grams of oxygen per hour.
Trudy Kortes, the director of technology demonstrations at NASA Headquarters in Washington DC, expressed the agency’s satisfaction in supporting such a technology, stating, “By demonstrating this technology in real-world conditions, we’ve moved one step closer to a future where astronauts can ‘live off the land’ on the Red Planet.”
MIT’s Michael Hecht, who leads the MOXIE development effort, mentioned in a statement that the team’s next focus will be on developing the larger version of MOXIE. Additionally, scientists will need to devise equipment for liquefying and storing the produced oxygen.
Robots and artificial intelligence are becoming an integral part of our daily experiences. They are involved in creating new medicines, answering queries (though sometimes inaccurately), and acting as personal digital assistants. Given sufficient time, they may permeate every aspect of our lives, from emotional understanding to space exploration. Just consult M3GAN, a cutting-edge Model 3 generative android created to be your closest companion.
M3GAN’s debut performance ended in chaos, which perhaps explains why the latest AI-driven robot from real-world laboratories is aimed at Mars. Recently, a research team led by Jun Jiang at the University of Science and Technology of China in Hefei unveiled an AI-equipped robot capable of generating oxygen from Martian materials. The findings from this mechanical chemist were published in the journal Nature Synthesis.
Discovering How to Create Oxygen from Martian Soil
As we advance to the next stage of human space exploration, there is significant emphasis on utilizing local materials at our destinations. Anything we can find or produce on the Moon, Mars, or any other celestial body is an asset we don’t need to launch from Earth’s gravity and haul with us. Among all resources, oxygen is crucial.
The robotic, AI-driven chemist resembles a large box, akin to a refrigerator positioned on its side. A robotic arm extends from one side, enabling the robot to handle various materials. Researchers provided the robot with five meteorites that originated from Mars or had compositions similar to Martian surface rocks, then allowed the robot to operate independently.
The robot employed acid and alkali to decompose the Martian ore and assess its components. After determining what resources were available, it examined 3.7 million potential combinations to identify a catalyst that would facilitate an oxygen-evolution reaction, releasing oxygen from water. Notably, it managed the entire process—preparing Martian materials, synthesizing the catalyst, characterizing it, conducting tests, and seeking the optimal formula—without any human intervention.
The team projected that the robot could generate 60 grams of oxygen per hour from a single square meter of Martian soil. Of course, this isn’t the sole experiment aimed at producing oxygen on Mars; NASA’s Mars Oxygen In-Situ Resource Utilization Experiment (MOXIE) aboard the Perseverance rover has already succeeded in producing oxygen from Martian air on the planet. Nonetheless, when venturing off Earth, having multiple tools for oxygen production is invaluable.
Additionally, the same robotic chemist system that successfully unveiled the method for extracting oxygen from Martian soil could potentially create various catalysts and compounds. The system’s strength lies not merely in its oxygen production ability but rather in its capacity to explore pathways toward any target compound using available materials. Provided, of course, that a viable chemical pathway exists.
It’s comparable to asking a skilled chef to prepare a pizza using random ingredients from your pantry and the back of your freezer. Mars lacks breathable oxygen, but it contains ample water ice at the poles and an almost unlimited supply of Martian rock elsewhere. As long as an AI-driven robotic chemist is available, those two ingredients are sufficient to produce all the breathable air we could need. We just hope that the robot doesn’t turn hostile when we require its help the most.
Mars and other planets present challenges for study due to their immense distance. But what if we could bring a piece of Mars to Earth, allowing scientists to analyze it without needing space suits? In a study published on Monday in Nature, researchers in China report the development of a “robotic artificial-intelligence chemist” that utilized machine learning to extract oxygen from Martian meteorites. The researchers aim to use their AI chemist bot to support a sustainable human presence on Mars.
Discovering signs of life on Mars or establishing our existence there has been one of humanity’s most cherished dreams for as long as we have recognized the existence of other planets. More conducive to life than the toxic smog of Venus, Mars appears to be the closest planet that could sustain life as we know it. But how could we—or any life—exist on Mars?
One hypothesis regarding the origin of life suggests that a single source may have “seeded” numerous planets with the templates from which living organisms could emerge. Evidence often cited in support of this idea includes lunar and Martian rocks that have reached Earth, propelled into space by volcanic eruptions or impact events.
These Martian rocks also represent an excellent opportunity to directly study the chemistry of the Red Planet without needing to travel there. This makes them highly valuable for research into in-situ resource utilization (ISRU), which proposes the use of materials from Mars (or other places) to establish a presence there rather than transporting everything from Earth. What better experimental ground than genuine rocks from Mars?
A project led by a multidisciplinary group of scientists in China aimed to create a middle ground for ISRU research: a self-sufficient research platform capable of functioning on Mars with minimal, if any, human oversight. They developed what they referred to in their paper as an “all-in-one robotic artificial-intelligence chemist,” which successfully generated oxygen from Martian meteorite samples as a proof of concept.
The vision is for the robot to collect Martian regolith samples and deduce solutions to specific problems using fundamental reasoning—without any human intervention. Place this device in a remote area of the Andes with no manual, and it could still identify which rocks would serve best as flint for igniting a fire. However, the amount of oxygen available on Mars is insufficient for combustion. Mars’ carbon dioxide atmosphere is only one percent of the pressure found in Earth’s breathable atmosphere at sea level. This makes extracting O2 from CO2 seem impractical. So, how and where would humans acquire the oxygen necessary for prolonged habitation on Mars?
Energy is limited and costly on Mars’ cold and arid surface. Nonetheless, Mars is rich in rusty, oxygen-bearing rocks. Recently, it has been discovered that, not too long ago, the Martian surface was unexpectedly wet. Water ice has been detected along the edges of craters and ravines on Mars. Therefore, the scientists considered the potential for a catalyst. However, the report indicates that from just five different Martian ores, over three million potential candidates emerged for a catalyst exhibiting two specific features: it must be made entirely from in-situ materials and must be effective at extracting oxygen from metal oxides in Martian meteorites, essentially “un-rusting” rust.
This is where AI plays a crucial role. Instead of employing trial and error, the team entrusted the research to the AI, which effectively identified the most promising candidates far quicker than humans could.
With the selected catalyst, the report describes a chemist-bot that utilized a low-power electrochemical bath, connected with pure silver and a platinum counter-electrode. By adding the meteorite samples to the saline electrolyte bath and activating the power, oxygen gas is released during the reaction, while metal ions accumulate, dissolved in the electrolyte. Once the oxygen has risen out of the solution, it becomes available to humans in its diatomic form.
The report does not clarify how well this process will scale. However, it suggests a future “workflow” that involves incorporating the de-oxidized metal samples into Nafion, a polymer adhesive, to create conductive circuits intended for purity testing or custom transistors printed on-site.
Even without the mention of AI and its related buzzwords (and the associated funding), the robot AI chemist is part of a commendable endeavor. Both public and private research institutions have announced significant advancements in ISRU within the last six months. During the summer, UK chemists accomplished the direct conversion of water into hydrogen and oxygen using sunlight, without the need to convert sunlight into electricity, showcasing a low-energy system. Furthermore, NASA’s recent ISRU experiments employed Earth-based analogs of regolith to serve as a substrate for creating “Marscrete” structures, as well as using a laser to convert actual regolith into carbon monoxide. NASA’s Perseverance Mars rover also carried the MOXIE in-situ oxygen generation experiment, which successfully produced a proof-of-concept amount of oxygen on Mars’ surface.
Chinese researchers have successfully used an AI-driven robot to autonomously create optimal catalysts for generating oxygen on Mars.
According to a report from the University of Science and Technology of China (USTC), the robot synthesized and optimized catalysts aimed at facilitating oxygen evolution reactions on Mars using five distinct Martian meteorites.
Recent findings of water on Mars have opened up possibilities for large-scale oxygen generation from water molecules through solar power-driven electrochemical processes, utilizing catalysts for oxygen evolution reactions.
Researchers at USTC disclosed that the AI robot utilizes a machine-learning model to determine the best catalyst formula from over 3.76 million potential compositions sourced from various Martian minerals.
The robotic chemist, referencing 50,000 chemistry research papers, managed to complete the intricate catalyst optimization in less than two months—a feat that would take approximately 2,000 years for a human chemist.
Experiments carried out at minus 37 degrees Celsius, simulating Martian temperatures, confirmed that the catalyst can reliably produce oxygen without visible deterioration on the Martian terrain.
The study confirms that the AI chemist can develop new catalysts, which could lead to significant progress in oxygen generation, infrastructure building, and food production on other planets, as well as facilitate the production of additional chemicals from Martian resources.
“In the future, humans could set up an oxygen production facility on Mars with the support of the AI chemist,” stated Jiang Jun, the project’s lead researcher.
He noted that just 15 hours of solar exposure would be adequate to generate the oxygen levels required for human survival.
“This groundbreaking technology brings us closer to realizing our aspiration of living on Mars,” added the professor.
On Monday, Chinese scientists introduced an artificial atmospheric model of Mars known as “GoMars.” This model is intended for use in China’s future Mars exploration missions planned for around 2028.
In recent years, Beijing has significantly invested in its space program, achieving milestones such as the Chang’e 4 lunar probe, which successfully landed on the Moon’s far side in January 2019.
Using meteorites from Mars, an AI-equipped robotic chemist synthesized compounds that could facilitate oxygen generation from water.
Future crewed missions to Mars will require oxygen not only for astronauts’ respiration but also for use as rocket fuel. A crucial aspect of making these missions economically viable over time is utilizing resources available on the Red Planet to generate oxygen, rather than transporting it from Earth.
This approach is promising since Mars has substantial reserves of frozen water ice. As water is composed of hydrogen and oxygen, scientists are exploring ways to extract the latter from these Martian water reserves. Specifically, catalysts can accelerate the chemical reactions that “split” water molecules to produce oxygen and hydrogen gas.
In a recent study, researchers utilized an AI chemist to develop some of those water-splitting catalysts, focusing on materials sourced from Mars. The team investigated five categories of Martian meteorites, which are rocks that have fallen to Earth after being ejected from the Red Planet by cosmic impacts.
The AI chemist employed a robotic arm to gather samples from the Martian meteorites and utilized a laser to scan the ore. It calculated over 3.7 million molecules that could be created using six different metallic elements present in the rocks—iron, nickel, manganese, magnesium, aluminum, and calcium.
In just six weeks, completely independently, the AI chemist chose, synthesized, and tested 243 different molecules. The most effective catalyst identified by the robot was able to split water at minus 34.6 degrees Fahrenheit (minus 37 degrees Celsius), the type of frigid temperature found on Mars.
“When I was a child, I dreamt of exploring the stars,” said Jun Jiang, co-senior author of the study and a scientist at the University of Science and Technology of China in Hefei, in an interview with Space.com. “So when we finally realized that the catalysts produced by the robot were capable of producing oxygen by splitting water molecules, I felt as if my dreams were becoming a reality. I even started to envision myself living on Mars in the future.”
The researchers estimate it would have taken a human scientist roughly 2,000 years to discover that “best” catalyst using traditional trial-and-error methods. However, Jiang acknowledged that while these findings indicate that AI can be a valuable asset in scientific endeavors, it “still requires the oversight of human scientists. The robot AI chemist is effective only if we have taught it what to do.”
The scientists now plan to investigate whether their AI chemist can function under additional Martian conditions beyond temperature.


